A growing body of evidence shows that your gut microbiome functions as your body’s second brain. In this case, however, the balance of gut flora directly affects your immune system and intestinal tract. Imbalances and deficiencies – also known as gut dysbiosis – can lead to inflammation and the development of autoimmune diseases, including Crohn’s disease, ulcerative colitis, psoriasis, lupus, rheumatoid arthritis, and multiple sclerosis.
Understanding the Gut Microbiome
Your body is home to thousands of bacterial species, with roughly two-thirds found in the intestinal tract. Its diversity and composition begin building from the moment you’re born, evolve over the lifespan, and eventually harbor a complex genome. As you grow older, the gut microbiome begins to affect your health, immune system response, metabolic function, and how your body draws in and utilizes nutrients and eliminates waste.
On a general level, the microbiome consists of beneficial microorganisms, potentially sensitive pathogens, and pathogenic bacteria. Imbalances of the latter two correlate with the development of multiple diseases. As well, what you eat, swift dietary changes, using probiotics, and taking antibiotics can all alter this balance.
When one microorganism or pathogen dominates the others either through deficits or an overabundance, your gut microbiome can:
- Influence how you metabolize food.
- Alter your immune system’s state of homeostasis.
- Increase your risks for infections.
- Break down or alter the intestinal barrier, increasing permeability and the potential for leaky gut syndrome.
- Contribute to the development of cardiovascular diseases and neurological disorders.
Gut Health and Autoimmune Diseases
For a baseline understanding, autoimmune diseases are characterized by your immune system attacking your body.
Ordinarily, your immune system responds when it notices bacteria, a virus or other microorganism present in your body, and generates antibodies as part of its defense mechanism. With autoimmune diseases, this process targets healthy cells and tissues. As the body of research around autoimmune diseases grows, multiple factors appear to trigger their development, including genetics, environmental exposures, infections and select medications that reroute the body’s immune response.
Behind this reaction is an over-production and abnormal production of B and T cells, along with inflammatory-encouraging cytokines. Research has found that changes in the gut microbiome, often in response to diet or antibiotic use, may influence the immune system’s response, ultimately causing it to produce high levels of cytokines, generate an imbalance in T cells and affect how the body perceives and responds to antigens, including both self-generated and foreign.
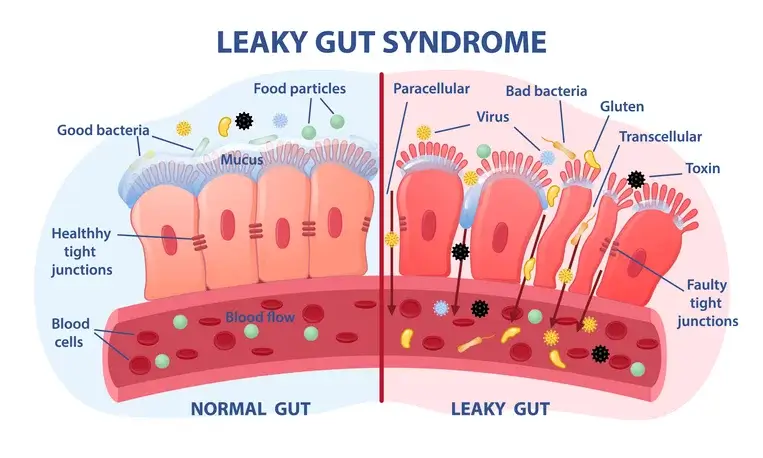
In terms of triggering an autoimmune disease, the gut microbiome may influence their development, often spurred through intestinal permeability and molecular mimicry. Research has found that individuals diagnosed with autoimmune disease tend to have an altered, if not more permeable, gut barrier, which increases exposure risks for the rest of the body. This may also be coupled with decreased tolerance from the immune system to the bacteria and pathogens present in the microbiome.
Integrative medicine practitioners often call this leaky gut syndrome, which allows bacteria and pathogens to pass through the intestinal wall and travel through the bloodstream throughout the rest of the body. This shift can lead to inflammatory bowel disease (IBD), Crohn’s disease, celiac, lupus, and multiple sclerosis and may even worsen diabetes.
How Gut Health Influences the Development of Autoimmune Diseases
Direct
A 2017 study published in BMC Immunology identified a correlation between gut dysbiosis and the development of autoimmune diseases, including IBD, type 1 diabetes and rheumatoid arthritis. This relationship appears to stem from a poorly regulated immune system in response to inflammatory microbes from the gut.
Research from Yale University examined the relationship between a permeable intestinal barrier and the development of autoimmune diseases. With some given an antibiotic treatment, mice as subjects had their lymph nodes, spleen and liver analyzed. Researchers noticed the presence of Enterococcus gallinarum in these areas, and they found that the bacteria could alter the gut barrier, pass to the lymph nodes and liver and aggravate an autoimmune reaction. They also noticed that the DNA present in E. gallinarum could mutate, causing the bacteria to congregate in the intestinal walls and eventually progress to the lymph nodes and liver.
They further observed that the bacteria could exist outside of the gut, undetected. Once being noticed, the immune system attempted to fight it through an inflammatory response. This inflammation then spread to other organs.
Research from the Queen’s University Belfast found a correlation between the development of autoimmune disorders and the presence of a “mimic protein” generated by Bacteroides fragilis, a microorganism found in the human gut microbiome that can also pass through the intestinal barrier. Its presence resulted in the immune system’s defense attacking the body’s own tissues. They noted that patients living with lupus or rheumatoid arthritis were more likely to have this microbe present than those not diagnosed with an autoimmune disease.
Indirect
Research has further attempted to determine why gut microbial imbalances occur and how these factors can trigger an immune response.
Studies have found that environmental factors may be to blame, including antibiotic and medication overuse and chemical exposures causing the gut microbiome to become less diverse. This pattern, in turn, leads to a weakened gut barrier, an increase in inflammation, and a potentially adverse immune system reaction.
A lack of diversity may also occur from birth. Researchers have found that children delivered through C-section, given antibiotics not long after, or for whom breastfeeding was delayed end up having an altered gut microbiome from the get-go.
Into adulthood, stress, what we eat, and inconsistent sleep patterns can encourage inflammation and transform the composition of the gut microbiome.
Turn to Functional Medicine to Improve Gut Health
Looking for a wellness-rooted approach to addressing gut health? Turn to CentreSpringMD. We’re a holistic primary care practitioner specializing in integrative family medicine. Interested in learning more? Contact us today to set up a virtual appointment.
Resources:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6854958/
https://bmcimmunol.biomedcentral.com/articles/10.1186/s12865-016-0187-3
https://www.nih.gov/news-events/nih-research-matters/gut-microbe-drives-autoimmunity
https://www.managedhealthcareexecutive.com/view/common-gut-bacteria-linked-autoimmune-diseases
https://www.tandfonline.com/doi/full/10.4161/gmic.19320
https://www.frontiersin.org/articles/10.3389/fmed.2018.00135/full
